Crude drug sample data base
※Click on the image to enlarge it.
The capital city, provincial capital city or the representative
location of its administrative area is indicated.
location of its administrative area is indicated.
30.592849
114.30553899999995
Production area information
People's Republic of China,Hubei Prov.
https://ethmed.toyama-wakan.net/img/pin_san.png
34.6937378
135.50216509999996
Collection information
Japan,Osaka Pref.
https://ethmed.toyama-wakan.net/img/pin_nyu.png
Scientific information data base
| Common name | 党参, Dangshen, Codonopsis Radix (JP18, CP2020), Codonopsis Root (JP18), Tangshen (CP2020) | ||||||
|---|---|---|---|---|---|---|---|
| crude drug image |
| ||||||
| Original plant name | Codonopsis pilosula Nannfeldt1 or Codonopsis tangshen Oliver, (Hikagetsuruninjin1) | ||||||
| original plant image |
| ||||||
| Family name | Campanulaceae | ||||||
| Used part | root | ||||||
| Official compendium | JP XVIII, CP (2020 ed.) | ||||||
| Clinical application | As a tonic, dangshen is applied for lack of appetite, exhaustion, cough and thirst. | ||||||
| Medical system | Traditional Chinese medicine | ||||||
| Drug effect in traditional medicine | Traditional classification | Drugs for replenishing Qi (vital energy) | |||||
| Beneficial effect | [Property and Flavor] Neutral; sweet. [Meridian Tropism] Spleen and lung. [Actions] To fortify the spleen and replenish lung, nourish blood and engender fluid. [Indications] Spleen-lung qi deficiency, reduced food intake, fatigue, cough and dyspnea of deficiency type, deficiency of qi and blood, sallow complexion, palpitations and shortness of breath, thirst caused by fluid consumption,wasting-thirst internal heat. | ||||||
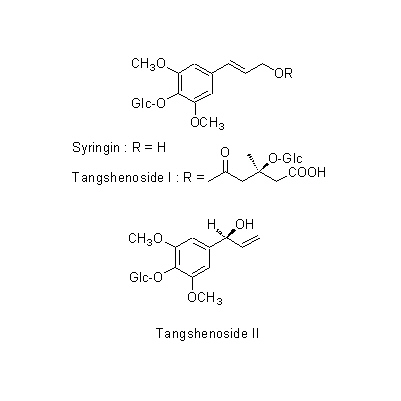
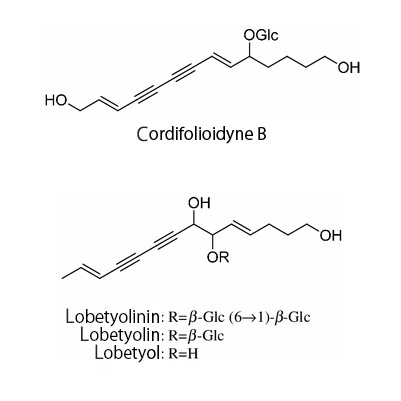
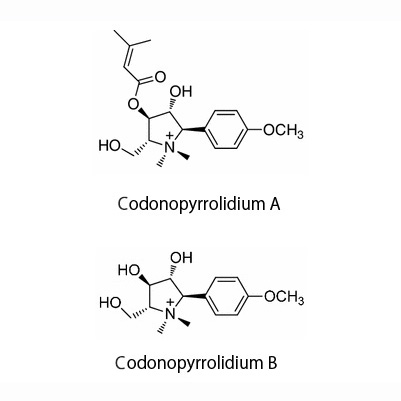
| Chemical constituent | Polyacetylene derivatives (*C2): Lobetyolin, Lobetyol, Cordifolioidyne B Phenylpropanoids (*C1): Syringin, Tangshenoside I, Tangshenoside II Triterpenoids (*C2): Codonopilate A, B, C Alkaloids (*C2): Codonopyrrolidium A, B Polysaccharides (*C1): Inulin Others (*C1): サポニン/Saponin, 粘液/Mucilage | ||||||
| Chemical structure |
※画像をクリックすると、拡大して表示されます。 | ||||||
| DNA sequence | AF107584, AF134859, AF134860, AF134861, AF136237, L18797, AF183445, AF183446, AF183443; Traditioal Medical & Parmaceutical Database. | ||||||
| Disease | Anorexia, Malaise, Muddy and watery stool, Shortness of breath, Dyspnea, Cough, Lightheadedness, Palpitation, Thirst | ||||||
| Formulation | |||||||
| Related drugs | Manjin | ||||||
| References | JP18: The 18th edition of the Japanese Pharmacopoeia. CP2020: Pharmacopoeia of the People's Republic of China 2020 edi. B1) Shoyakugaku Zasshi,46,156(1992); 46,165(1992); 46,217(1992); 46,358(1992). B2) PMID:24203345. B3) PMID:23765107. C1) Outline of Pharmacognosy, a Textbook, p221. C2) Parter Shoyakugaku, pp309-310. | ||||||
| Remarks | The Pharmacopoeia of The People's Republic of China lists C. pilosula, C. pilosula var. modesta and C. tangshen as Dangshen. In addition to these, the followings are also used: C. subglobosa W.W.Sm. (in Sichuan Prov.); C. tubulosa Kom. (in Sichuan, Guizhou and Yunnan Prov.); C. nervosa Nannf. and C. canescens Nannf. (in Sichuan, Xizang Autonomous Region); C. clematidea C.B.Clarke (in Xinjiang Uygur Autonomous Region). These days in China, "Dangshen" is popular as a substitute drug for Ginseng. The name of Dangshen can be dated back to Bencaocongxin of the Qing Dynasty. Since then, there have been two theories about the different medical effects between Dangshen and Ginseng. One theory says "Though Dangshen has a benefit of clearing away the lung heat, doctors apply it in substitution for Ginseng so easily and only make the disease worse." The other says "The benefit of Dangshen is equal to that of ginseng." There are some problems about the equality of the medicinal effects. | ||||||
| Last renewal date | 2021/09/27 | ||||||