Crude drug sample data base
※Click on the image to enlarge it.
Crude drug name Market name GRANATI CORTEX Formal name 石榴幹皮 Japanese name せきりゅうかんぴ, Sekiryūkampi Vernacular name Shiliuganpi Latin name Granati Cortex English name Pomegranate Bark Original plant name Punica granatum L., (Zakuro)Family name Punicaceae Used part Classification Plant origin Sub classification bark TMPW No. 2409
Scientific information data base
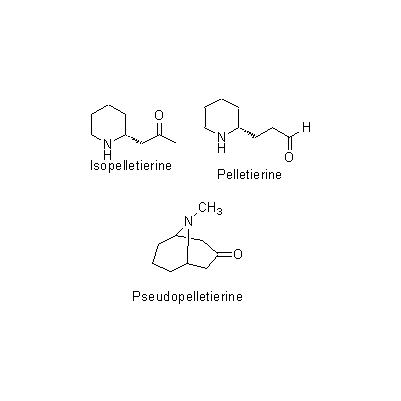
Common name 石榴幹皮,Granati Cortex, Pomegranate Bark Synonyms 石榴皮 Original plant name Punica granatum Linn., (Zakuro)original plant image Family name Punicaceae Used part bark Clinical application It is used against tapeworms especially armed tapeworms as an anthelmintic. It is also the raw material for pelletierine tannate. Medical system Traditional Chinese medicine Drug effect in Traditional Anthelmintics Chemical constituent Tannins Chemical structure Pharmacological effect Anthelmintic (decoction, total alkaloid, pelletierine: ameba, leukocyte, paramecium, leech, worm, tape worm), suppression of bacteria (water leaching: gram-negative bacteria, cutaneous mycosis). DNA sequence U38311, L10223 Classical reference Disease Intestinal parasite, Abdominal pain Formulation not used in formula Related drugs Granati Radicis Cortex, Granati Pericarpium References C1)The Encyclopedia of Wakan-Yaku with Color Pictures Vol. Ⅱ, pp 134-135. Remarks The pericarp of Punica granatum L. (Jap. name: Zakuro) is used as an astringent and anti-diarrheal. The root bark or bark is processed to make vermifuge for pork tapeworm. When it is used as a vermifuge, it should be soaked in water 30 to 50g/day and taken by decoction (3 to 4 times in an hour). Excess use causes side effects such as vomiting, dizziness, nausea, and diarrhea. Last renewal date 2020/12/22