Crude drug sample data base
※Click on the image to enlarge it.
The capital city, provincial capital city or the representative
location of its administrative area is indicated.
location of its administrative area is indicated.
Production area information
https://ethmed.toyama-wakan.net/img/pin_san.png
27.7172453
85.3239605
Collection information
Kingdom of Nepal,Kathmandu
https://ethmed.toyama-wakan.net/img/pin_nyu.png
Scientific information data base
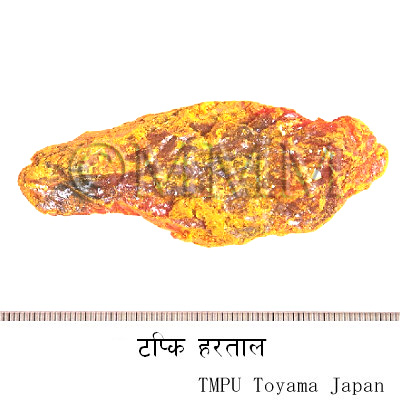
| Crude drug name | Ayurvedic name or Sanskrit name, English name | Haritala | |||
|---|---|---|---|---|---|
| Synonyms | Tala, Ala, Talaka. | ||||
| crude drug image |
| ||||
| Original plant name | Trisulphate of arsenic | ||||
| Used part | Purified bhasma | ||||
| Therapeutic uses | Kandu (pruritus), Kustha (skin diseases), Asyaroga (diseases of the mouth), Vrana (ulcers). | ||||
| Medical system | Ayurveda (Traditional Indian medicine) | ||||
| Traditional concept | Rasa (Taste) | Katu (Pungent), Kasaya (Astringent) | |||
| Virya (Potency) | Usna (Hot) | ||||
| Guna (Quality) | Laghu (Light), Snigdha (Unctuous) | ||||
| Vipaka (Post digestive taste) | Katu (Pungent) | ||||
| Karma (General action) | Visahara (pacifies poison) | ||||
| Dosakarma (Action on dosa) | Decreases Kapha Vata | ||||
| Formulation | Rasamanikya, Talakesvara rasa, Vidyadhara rasa. | ||||
| Comments | Two varieties of Haritala have been mentioned in the classics as 1.Patra haritala (flaky form), 2.Pinda haritala (lumpy form). Patratala variety is said to be tridosaghna (pacifies all the dosas) and rasayana (rejuvenative). Pindatala is said to be of inferior quality when compared to patratala. Talaka being a poisonous drug has to be used only after meticulous purification. There are many methods of purification. One of them is as follows: Haritala is powdered and tied in a potali (bolus). It is given svedana in dolayantra containing tilaksarajala for three hours. Then it is dried. | ||||
| References | Reference book Tips! | Dravyagunavijnana, Vols. 1-5, reprint 1998. Sharma, P.V., Chowkhambha Bharati Academy, Varanasi Vol. 3, pp 84-85 | |||
| Last renewal date | 2022/06/16 | ||||