Crude drug sample data base
※Click on the image to enlarge it.
Scientific information data base
| Common name | センナ, Sennae Folium (JP18, CP2020), Senna Leaf (JP18, CP2020) | ||||||
|---|---|---|---|---|---|---|---|
| Synonyms | 番瀉葉 (Fanxieye) | ||||||
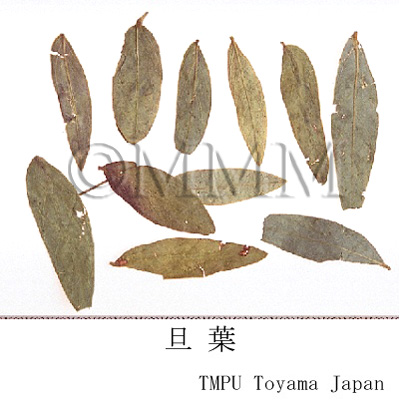
| crude drug image |
| ||||||
| Original plant name | Cassia angustifolia Vahl or Cassia acutifolia Delile, (Tinnevelly senna, Alexandria senna) | ||||||
| original plant image |
| ||||||
| Family name | Leguminosae | ||||||
| Used part | leaflet | ||||||
| Quality for selection | Good Senna contains few foreign material such as leaf rachis and petiole. (TN) | ||||||
| Official compendium | JP XVIII, CP (2020 ed.) | ||||||
| Clinical application | Laxative. Senna promotes digestion as an amaroid stomachic at low doses. It has a laxative effect at proper quantity (3~6g/day) and cures stagnation of undigested food, abdominal distention and constipation. It is especially used for heat-type constipation, and must not be used for diarrhea due to pathogenic cold. | ||||||
| Medical system | Traditional Chinese medicine | ||||||
| Drug effect in traditional medicine | Traditional classification | Purgatives, cathartics | |||||
| Beneficial effect | [Property and Flavor] Cold; sweet and bitter. [Meridian Tropism] Large intestine meridian. [Actions] To purge heat, move stagnation, open the bowels, and induce diuresis. [Indications] Heat bind, accumulation, stagnation, constipation with abdominal pain, edema distention and fullness. | ||||||
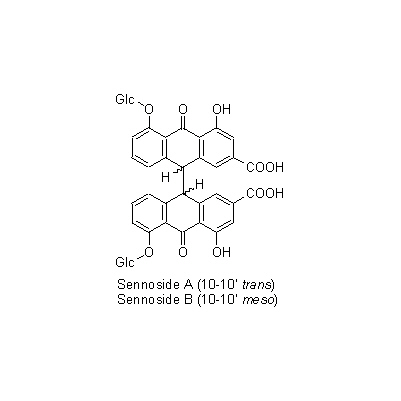
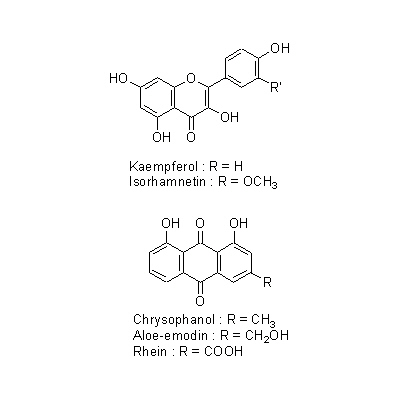
| Chemical constituent | Anthraquinones (*C1-C11): Sennoside A, Sennoside B, Rhein-aloe-emodin dianthrone diglucoside, Sennoside C, Sennoside D, Sennoside G, Aloe-emodin-dianthrone diglucoside, Rhein anthrone diglucoside, Chrysophanol, Aloe-emodin anthrone diglucoside, Rhein, Aloe-emodin-8-glucoside, Rhein 8-glucoside, 以下代謝物 / The following compounds are metabolites 8-Glucosylrheinanthrone, Rheinanthrone, 8-Glucosylrhein, Sennidin A-8-monoglucoside, Sennidin B-8-monoglucoside, Sennidin B-8'-monoglucoside, 8-Glucosylrheinanthrone Flavones & Flavonols (*C1): Kaempferol, Kaempferin, Isorhamnetin Naphthalenes C. angustifolia (*C12): 6-Hydroxy musizin glucoside C. acutifolia (*C12): Tinnevellin glucoside | ||||||
| Chemical structure |
※画像をクリックすると、拡大して表示されます。 | ||||||
| Pharmacological effect | Hyperperistalsis.Dianthrones such as sennoside A are metabolized into rheinanthrone by intestinal bacterium and have the cathartic effect. | ||||||
| DNA sequence | U74195 | ||||||
| Disease | Constipation, Full stomach, Abdominal pain, Ascites | ||||||
| Formulation | Compound Rhubarb and Sennna Powder | ||||||
| Related drugs | Senna pod (Sennae Fructus) | ||||||
| References | JP18: The 18th edition of the Japanese Pharmacopoeia. CP2020: Pharmacopoeia of the People's Republic of China 2020 edi. C1)The Encyclopedia of Wakan-Yaku with Color Pictures Vol. II, pp 95-97. C2)Outline of Pharmacognosy, a Textbook, p 283. C3)Planta Med.,40,225(1980). C4)Chem.Pharm.Bull.,30,1338(1982). C5)J.Pharmaco-bio-Dyn.,8,800(1985). C6)Chem.Pharm.Bull.,35,1998(1987). C7)Pharmacology,36,172(1988). C8)Appl.Envir.Microbiol.,60,1041(1994). C9)Biol.Pharm.Bull.,19,701(1996). C10)Biol.Pharm.Bull.,19,705(1996). C11)Biol.Pharm.Bull.,19,136(1996). C12)Planta Med.,43,11(1981). | ||||||
| Remarks | Senna is listed in The Ebers Papyrus which is said to be the oldest medical document in the world. Yahya ben Masawaih, a court physician of an Arabian Calipf in 11th century, introduced it to Europe as a substitute laxative for Aloe. After that, Senna became a common laxative in Europe and the United States. In Japan, It has been listed in the Japanese Pharmacopoeia since the 1st edition. There are two kinds of Senna. One is Alexandrian Senna which is cultivated in the midstream area of the Nile. The other is Tinnevelly Senna which is cultivated in the states of Kerala and Madras, India. Japan imports Tinnevelly Senna. In Europe and the United States, mostly, Alexandrian Senna and its fruit (Senna Pod) are used. In China, it became a medicinal plant much later. It first appears in Chinese literature, "Yinpianxincan". | ||||||
| Last renewal date | 2025/01/09 | ||||||